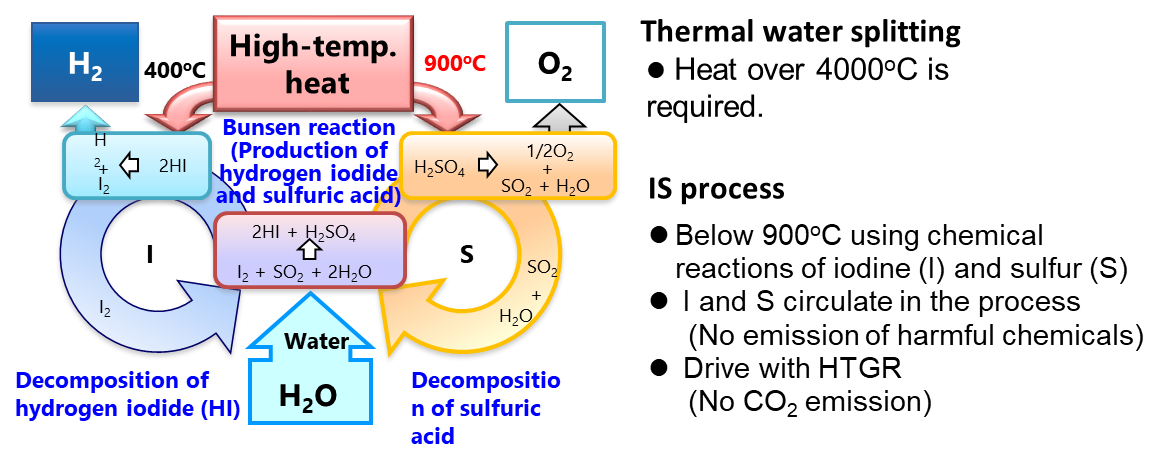
IS Process Research and Development is conducted R&D on hydrogen production technology using a high temperature gas-cooled reactor which is a promising candidate for next generation energy source. The hydrogen production technology named IS process is thermochemical water splitting chemical process using chemical compounds of iodine (I) and sulfur (S).
The world's energy system depends on finite and uneven distribution fossil fuel resources. The hydrogen utilization like a fuel call car can be a candidate to solve this problem. In order to realize the hydrogen energy economy, all of advantage, which is suitability for mass production, economical, and environmental friendly, is essential for the hydrogen production technologies, raw materials, and primary energy. In recent years, water splitting hydrogen production methods powered by nuclear energy and renewable energy have much attention to satisfy all of these requirements.
This group is engaged in research and development of the IS process. First, at the laboratory stage, 1L/h of hydrogen production was accomplished for 24 by finding inhibition factors (side reactions etc.) and appropriate reaction conditions (temperature, composition). At the next basic engineering stage, a plant control technology, which almost automates the operation of hydrogen production, was developed for stable hydrogen production. Continues hydrogen production of 30L/h for 1 week was carried out keeping the amount of hydrogen and oxygen generated at a stoichiometric ratio (2: 1).
Looking at water splitting hydrogen production, the water electrolysis is known as common technology; the existing water splitting hydrogen production consumes electric energy. Unlike the electrolysis, the thermochemical water splitting methods requires heat energy to produce hydrogen by combining a plurality of chemical reactions, which enables one step energy form conversion (heat ->hydrogen) contrasted to the electrolysis of two step (heat ->electricity ->hydrogen), so the thermochemical methods have a possibility to produce hydrogen with high thermal efficiency. Among many thermochemical hydrogen production methods, currently the most active research and development have been studied on IS process; I (iodine element symbol) and S (sulfur element symbol) are used for consisted chemical reactions.
Since the IS process converts thermal energy into chemical energy of hydrogen, improvement of thermal efficiency is one of important tasks. In order to reduce required heat to separate hydrogen iodide (HI) from Bunsen reaction solution, a method to pre-concentration HI solution is devised by using electro-electro-dialysis with cation exchange membrane; the concentration performance was confirmed experimentally. Membrane R&D to improve selectivity and proton conductivity is under way by introducing radiation graft polymerization technology.
At the elemental technology development stage, we promoted R&D on improvement of thermal efficiency and development of anti-corrosion and heat-resistant equipment because the IS process handles very corrosive process fluids (sulfuric acid and halogen such as iodine).
In 2010 - 2014, the soundness verification stage, chemical reactors that can withstand the actual environment (corrosive environment, high temperature environment) were developed using practical equipment materials. Besides, process data enabling hydrogen production efficiency of 40% were acquired on the electro-electro dialysis processing.
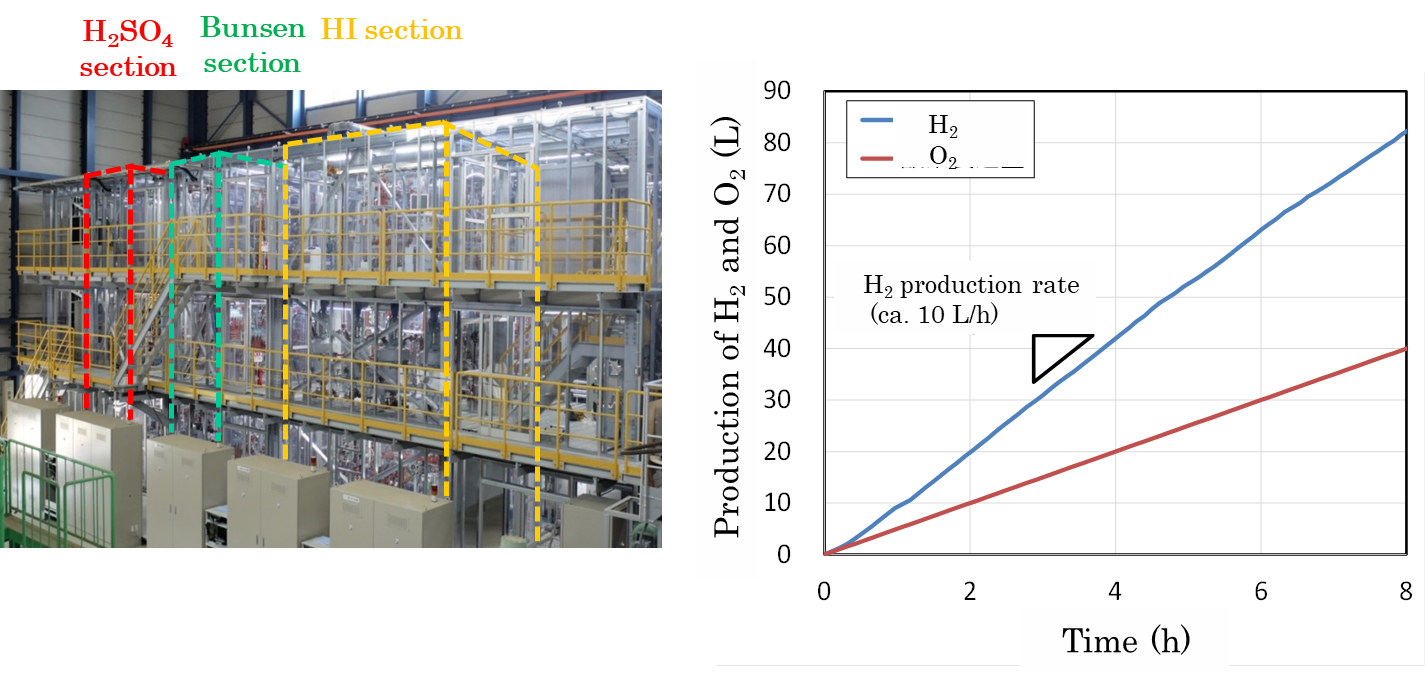
The IS process consists of three chemical reactions (Bunsen reaction: the formation of sulfuric acid and hydrogen iodide from water, sulfuric acid decomposition reaction: oxygen from sulfuric acid, hydrogen iodide decomposition reaction: production of hydrogen from hydrogen iodide). With consideration to the environment for each of these three chemical reactions, a Bunsen reactor using corrosion resistant lining (fluororesin plastic), a sulfuric acid decomposition reactor made of SiC ceramics for high temperature, and a hydrogen iodide decomposition reactor made of nickel base alloy for high temperature were fabricated and their soundness were shown using test equipment (hydrogen production scale: 100 liters of hydrogen per hour) made of industrial materials in actual three chemical reaction steps.
Recently, we are working on verifying the soundness of plant-wide equipment and hydrogen production performance, etc. since 2004. Firstly, gastight function, gas distribution function, liquid flow and heating were confirmed as basic functions checking. After that, the processing rate data in each reactor (hydrogen production by HI decomposition reactor etc.), separation and gasification function (HI gas distillation etc.), were acquired. The entire process connecting the three process sections was operated in hydrogen production for 8 hours at rate of 10L/h in Feb. 2014, and in Oct. operation was extended to 31 hours at twice the hydrogen production rate (20L/h).
Through these operations, technical issues were obtained that prevention of clogging and stable control of solution composition in Bunsen reaction process are important for next longer operation. Currently, in order to achieve longer-term stable operation and to confirm the operation procedure including start-up and shutdown, improvements of equipment are under way. We are also planning HTTR heat utilization tests (HTTR-GT / H2 test) are planned in the future to establish safety standards and design measures for when connecting nuclear reactor and hydrogen production facilities.