
February 26, 2015
Japan Atomic Energy Agency
National Agriculture and Food Research Organization
[Key points]
A research group at the Quantum Beam Science Center of Japan Atomic Energy Agency (JAEA; president, Shojiro Matsuura.) in collaboration with the NARO Institute of Floricultural Science of National Agriculture and Food Research Organization (NARO; president, Tokio Imbe.) succeeded in determining the crystal structures of a glucosyltransferase1) involved in the biosynthesis of blue anthocyanins2) which are known as natural pigments concerned in determining the color of flowers.
Ct3GT-A3) is an enzyme found in the petals of Clitoria ternatea (Fig. 1A) and is involved in an initial glucosylation step toward the biosynthesis of anthocyanin pigments designated as ternatins (Fig. 1B). Although the crystal structures of several glycosyltransferases have been determined, the structural information of acceptor-substrate complexes was limited presumably because of the instability of anthocyanidins4) (Fig. 2). In this research, the crystal structures of Ct3GT-A in complex with anthocyanidins, stabilized under weakly acidic conditions, were determined using synchrotron radiation at the High Energy Accelerator Research Organization (KEK) Photon Factory5) and at the SPring-86). The enzyme recognition of unstable anthocyanidins was firstly observed in this structural determination (Fig. 3A, 3B, and 3C), providing insight into anthocyanidin-binding configurations in this enzyme and a molecular mechanism for acceptor-substrate recognition.
This research achievement would be important for understanding how the characteristic blue pigments are produced in C. ternatea. Since this structural analysis revealed the detailed interactions between the enzyme and acceptor substrates, the structural information would be utilized to develop genetically engineered enzymes and to produce novel organic pigments or pharmaceutical candidates.
This achievement was published online in the Protein Society’s journal “Protein Science” on February 24, 2015.
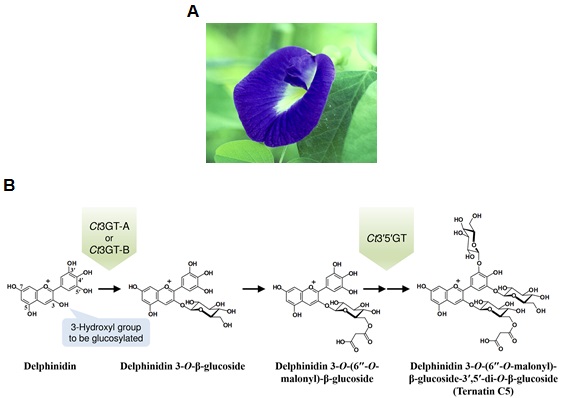
Fig. 1 (A) Flower of C. ternatea (butterfly pea). (B) Early biosynthetic pathway of anthocyanin pigments designated as ternatins in C. ternatea. The delphinidin aglycone, which is one of the typical anthocyanidins, is converted into ternatin C5 through glucosylation by Ct3GT-A, Ct3GT-B, and Ct3′5′GT.
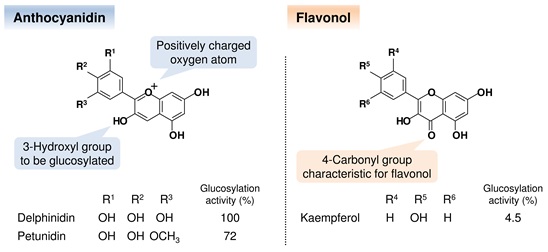
Fig. 2 Chemical structures of the acceptor substrates and the glucosylation activities for Ct3GT-A. The substituent pattern on the single ring influences the color appearance of each glucoside. The glucosylation activities for petunidin and kaempferol were normalized against that of delphinidin.
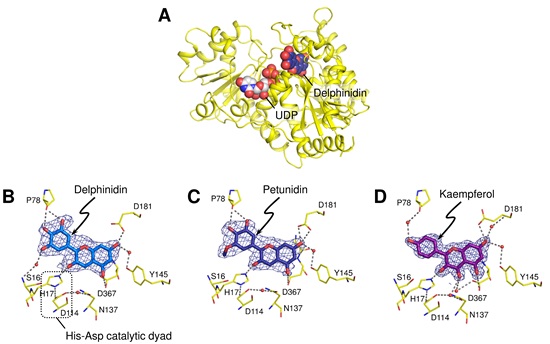
Fig. 3 (A) Overall structure of Ct3GT-A. The UDP molecule was modeled into the donor-binding site of the delphinidin-bound form of Ct3GT-A. The close-up views of the delphinidin binding (B), petunidin binding (C), and kaempferol binding (D) are shown. The kaempferol molecule was situated in the binding site in a manner similar to that seen in the delphinidin- and petunidin-bound forms, although the glucosylation activities against flavonols were significantly low. It suggests that the activities depend on the deprotonation efficiency of the hydroxyl group to be glucosylated.
Glucosyltransferase is an enzyme that catalyzes the transfer of the glucosyl moiety from an activated nucleotide sugar, known as the “donor” substrate, to a nucleophilic glucosyl “acceptor” molecule, the nucleophile of which can be oxygen-, carbon-, nitrogen-, or sulfur-based.
Anthocyanin is one of natural pigments located in all tissues of higher plants, including flowers, leaves, roots, and fruits, and serves to produce their characteristic color appearing red, purple or blue. Anthocyanin is a general term for the glucoside counterpart of anthocyanidin.
“Ct3GT-A” is an abbreviation of UDP-glucose: anthocyanidin 3-O-glucosyltransferase found in the petals of C. ternatea. The homologous enzymes to Ct3GT-A, identified in the same plant, were designated as Ct3GT-B and Ct3′5′GT based on each glucosylation activity.
Anthocyanidin is a general term for the tricyclic compound based on flavylium cation which is a type of oxonium form (Fig. 2). The different substitution patterns on the single ring of anthocianidins affect their color appearance.
PF in KEK, located in Tsukuba City, Ibaraki Prefecture, Japan, is the first synchrotron light source in Japan, which produce the X-ray regions. In this research, the PF Structural Biology Beamline (BL6A) was used for collecting the X-ray diffraction data.
SPring-8, located in Harima Science Park City, Hyogo Prefecture, Japan, is a large synchrotron radiation facility, which delivers the most powerful synchrotron radiation currently available. The name “SPring-8” is derived from Super Photon ring-8 GeV (8 giga electron volts, being the power output of the ring). In this research, the Structural Biology Beamline III (BL38B1) was used for collecting the X-ray diffraction data.
[ BACK ]