 |
Press Releases |
 |
Press Releases |
HOME > News & Information > Press Releases
Oct. 20, 2011
Joint research group of JAEA and Japan Synchrotron Radiation Research Institute (JASRI) has clarified that a covalent-like nature of an aluminum hydride, which is attracting attention as hydrogen-storage materials, is important to the chemical bond between the hydrogen and the aluminum atoms by using synchrotron radiation soft x-ray.
Hydrogen is an ultimate source of clean energy, and a key to utilize the hydrogen energy is to explore hydrogen-storage materials in which a large amount of hydrogen can be absorbed and released regularly under ambient pressures and temperatures. Aluminum hydrides, which are lightweight in the hydrogen-storage materials, can store a large amount of hydrogen, and as a demerit high temperature and pressure are required for the hydrogen storage. To control the temperatures and pressures, it is essential to understand the bonding state of aluminum and hydrogen atoms in the aluminum hydrides, and it has been an unsettled problem.
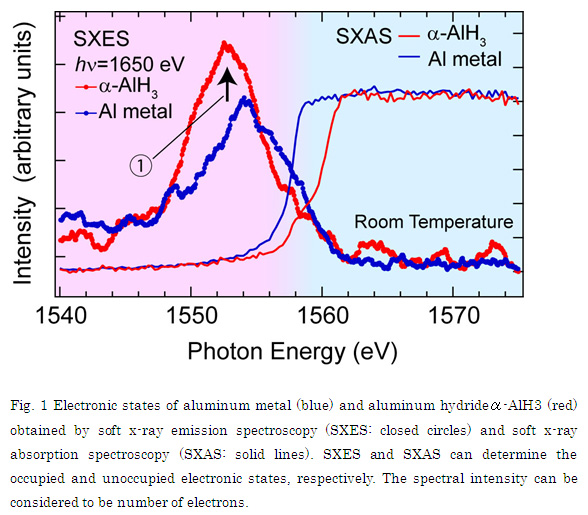
In this study, the research group determined the electronic occupied and unoccupied states in both aluminum metal and aluminum hydride by soft x-ray emission spectroscopy and soft x-ray absorption spectroscopy at SPring-8, and thus found out the difference of the electronic states before and after the hydrogenation (Fig. 1). As a result, it turns out that the electron number of aluminum atoms is increased in the aluminum hydride (shown as arrow 1). The observed result is an opposite behavior if the chemical bond is completely ionic. From the experimental evidence, it has been clarified that the aluminum hydride has a covalent-like bond between the aluminum and the hydrogen atoms.
The clarification of the aluminum-hydrogen bonding state will not only contribute to understanding the hydrogen-storage and release processes of aluminum hydrides but also provide directions for the design of new hydrogen-storage materials based on lightweight and inexpensive aluminum.
This work was supported by New Energy and Industrial Technology Development Organization (NEDO) under ďAdvanced Fundamental Research Project on Hydrogen Storage MaterialsĀE
These findings have been published in "Physical Review B" as an Advanced Online Publication on October 10th 2011.
Reference Figure
[ BACK ]