|

Success in Observation of Oxyanion Hole in Serine Protease
-Towards elucidation for catalytic mechanism of protease by neutron diffraction-
Jul. 30, 2009
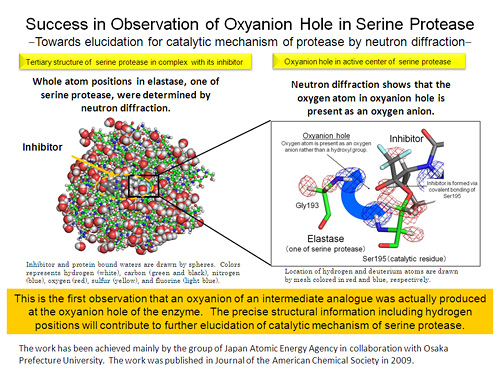
Almost one-third of all proteases can be classified as serine proteases, named for the nucleophilic serine residue at the active site. Three amino acids, histidine, aspartic acids, and serine (His57, Asp102, and Ser195; residues are numbered based on homology to chymotrypsin), compose the catalytic triad conserved in the active site of serine proteases. The nucleophilicity of Ser195 is hypothesized to increase via a �low-barrier hydrogen bond� (LBHB) formed between the side chains of His57 and Asp102. The tetrahedral intermediate is electrostatically stabilized though hydrogen bonds with the backbone amides of Gly193 and Ser195, together forming an �oxyanion hole�. However, the LBHB hypothesis in serine protease catalysis is still a matter of debate, and the state of oxygen atom of substrate in oxyanion hole has not been comfirmed yet. To help resolve long-standing questions regarding the catalytic activity of the serine proteases the structure of porcine pancreatic elastase (PPE) has been analyzed by high-resolution neutron crystallography because neutrons strongly interact with hydrogen and deuterium atoms, and neutron scattering lengths of hydrogen and deuterium atoms are very similar to those of carbon, nitrogen and oxygen atoms.
Results
(1) We have succeeded in preparing a large crystal of PPE in complex with a transition-state analog inhibitor FR130180.
(2) The structure of PPE in complex with FR130180 was determined by combined neutron crystallography at 1.65 � resolution and X-ray crystallography at 1.2 � resolution.
(3) The hydrogen bonding hydrogen between His57 and Asp102 is 0.96 � from the histidine nitrogen. This is not consistent with a LBHB which is predicted to have the hydrogen midway between the donor and acceptor atom.
(4) The neutron analysis also shows that the oxygen of the oxopropyl group of the inhibitor is present as an oxygen anion rather than a hydroxyl group, supporting the role of the oxyanion hole in stabilizing the tetrahedral intermediate in catalysis.
This is the first case that an oxyanion of an intermediate analogue is directly observed at an oxyanion hole. The precise structural information including hydrogen positions will contributes to further elucidation of catalytic mechanism of serine protease. In addition, the structural information including hydrogen positions will provide additional knowledge to improve inhibitor design for therapeutic application of fatal disease pancreatitis.
|

Location of environment monitoring posts measuring amount of
radiation. (details)

International link directory of related websites.
|
